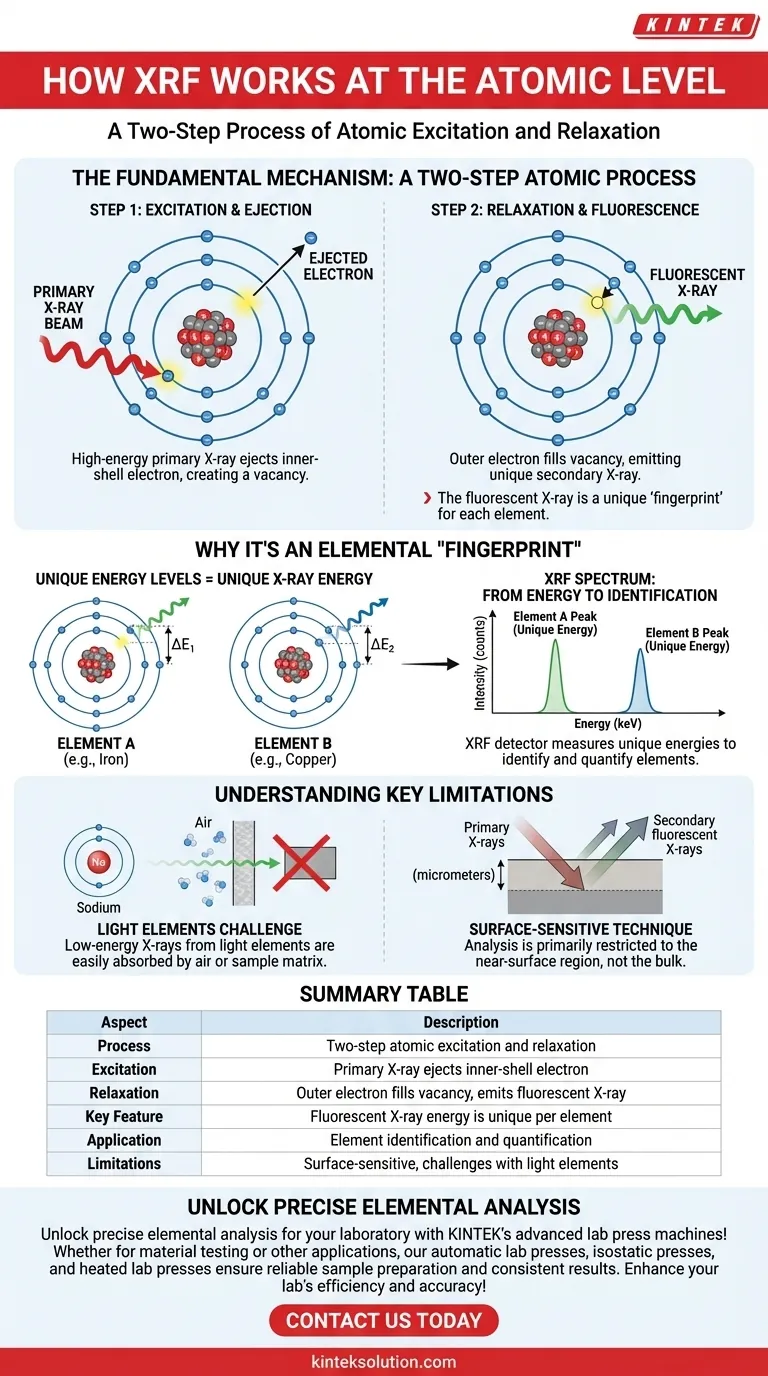
At its core, X-Ray Fluorescence (XRF) is a two-step process of atomic excitation and relaxation. A primary, high-energy X-ray beam strikes an atom in your sample, dislodging an electron from one of its inner shells. This creates an unstable vacancy, which is immediately filled by an electron from a higher-energy outer shell. To make this downward transition, the outer electron must shed its excess energy by emitting a secondary X-ray, which is the "fluorescence" that the instrument measures.
The essential principle is that the energy of this secondary, fluorescent X-ray is not random—it is a unique and predictable "fingerprint" for each element. By measuring these distinct energy signatures, XRF allows for the precise identification and quantification of the elements within a sample.

The Fundamental Mechanism: A Two-Step Atomic Process
To truly grasp how XRF works, we must visualize the events happening within individual atoms. The entire process hinges on the well-defined energy levels, or "shells," that electrons occupy around an atom's nucleus.
Step 1: Excitation and Ejection
The process begins when the XRF instrument fires a beam of primary X-rays into the sample.
These high-energy photons travel into the material and collide with atoms. If a primary X-ray has sufficient energy, it can transfer that energy to an electron in one of the innermost shells (typically the K or L shell).
This energy transfer ejects the electron from the atom entirely. The result is an atom in an unstable, excited state, now carrying a positive charge and a vacancy, or "hole," in its inner electron shell.
Step 2: Relaxation and Fluorescence
An atom cannot remain in this high-energy, unstable state for long. It naturally seeks to return to a more stable, lower-energy state.
To do this, an electron from a higher-energy outer shell (such as the L or M shell) immediately "falls" down to fill the vacancy in the inner shell.
Electrons in outer shells possess more energy than those in inner shells. As the electron drops to the lower-energy shell, it must release this energy difference. This released energy takes the form of a secondary X-ray photon, also known as a fluorescent X-ray.
Why This Process Creates an Elemental "Fingerprint"
The usefulness of XRF as an analytical technique stems from the fact that this fluorescent energy is unique to each element. This uniqueness is governed by the fundamental laws of atomic physics.
The Uniqueness of Electron Shell Energies
Every element is defined by the number of protons in its nucleus. This positive charge dictates the binding energy that holds each electron in its specific shell.
Because elements like iron, nickel, and copper have different numbers of protons, the energy gap between their respective K and L shells is different for each one.
From Energy to Identification
The energy of the emitted fluorescent X-ray is precisely equal to the energy difference between the electron's starting (outer) shell and its final (inner) shell.
Since this energy gap is a fixed, characteristic value for each element, the energy of the secondary X-ray serves as an unambiguous signature.
An XRF spectrometer's detector is designed to count these secondary X-rays and measure their specific energies. The output is a spectrum showing energy peaks that directly correspond to the elements present in the sample. The intensity of each peak generally correlates to the concentration of that element.
Understanding the Key Limitations
While powerful, the atomic principles behind XRF also create inherent limitations that every analyst must understand to interpret results correctly.
The Challenge of Light Elements
For light elements (e.g., Sodium, Magnesium, or Carbon), the energy of the fluorescent X-rays is very low.
These low-energy X-rays are easily absorbed by the air between the sample and the detector, or even by the sample itself (a phenomenon known as the matrix effect). This makes them difficult or impossible to detect with standard XRF instruments, often requiring a vacuum environment for analysis.
A Primarily Surface-Sensitive Technique
The primary X-rays can only penetrate a finite depth into the sample (from micrometers to millimeters, depending on the material). Furthermore, the secondary fluorescent X-rays can only escape from a limited depth before being absorbed.
This means XRF is fundamentally a surface-sensitive technique. The results accurately reflect the composition of the near-surface region, which may not be representative of the bulk material if the sample is not homogeneous.
Making the Right Choice for Your Goal
Your understanding of this atomic process directly informs how you should approach your analysis and interpret your data.
- If your primary focus is qualitative identification: Your goal is to detect the energy peaks, as the position of each peak on the energy spectrum directly corresponds to a specific element.
- If your primary focus is quantitative analysis: You must recognize that while the intensity (height) of a peak relates to concentration, it can be influenced by matrix effects from other elements and requires careful calibration.
- If you are analyzing light elements or thin films: You must be aware of the physical limitations of X-ray absorption and penetration depth, which are direct consequences of the energies involved in the atomic fluorescence process.
Understanding this atomic-level dance of excitation and relaxation transforms XRF from a black box into a predictable and powerful analytical tool.
Summary Table:
| Aspect | Description |
|---|---|
| Process | Two-step atomic excitation and relaxation |
| Excitation | Primary X-ray ejects inner-shell electron |
| Relaxation | Outer electron fills vacancy, emits fluorescent X-ray |
| Key Feature | Fluorescent X-ray energy is unique per element |
| Application | Element identification and quantification in samples |
| Limitations | Surface-sensitive, challenges with light elements |
Unlock precise elemental analysis for your laboratory with KINTEK's advanced lab press machines! Whether you're using XRF for material testing or other applications, our automatic lab presses, isostatic presses, and heated lab presses ensure reliable sample preparation and consistent results. Enhance your lab's efficiency and accuracy—contact us today to discuss how our solutions can meet your specific needs and drive your research forward!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press Lab Pellet Press Machine
- Manual Laboratory Hydraulic Press Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press Button Battery Press
- 24T 30T 60T Heated Hydraulic Lab Press Machine with Hot Plates for Laboratory
- Manual Laboratory Hydraulic Pellet Press Lab Hydraulic Press
People Also Ask
- What safety precautions should be taken when operating a hydraulic pellet press? Ensure Safe and Efficient Lab Operations
- What pressure range is recommended for pellet preparation? Achieve Perfect Pellets for Accurate Analysis
- What is the recommended pressing force for KBr pellets? Achieve Clear IR Spectroscopy Results
- What are the advantages of automated presses for XRF pellet preparation? Boost Lab Efficiency and Accuracy
- What are some specialized applications of hydraulic pellet presses? Enhance Precision in Catalyst and Battery Material Development



















