At its core, X-ray fluorescence is a method of elemental fingerprinting. The process uses a primary X-ray beam to energize the atoms within a sample, causing them to emit a secondary, "fluorescent" X-ray. The energy of this emitted X-ray is unique to each element, allowing for a rapid and accurate determination of a material's elemental composition without destroying it.
XRF doesn't just identify elements; it measures the unique energy signatures that atoms release when disturbed. This non-destructive process provides a reliable elemental breakdown of a sample, making it an invaluable analytical tool across science and industry.

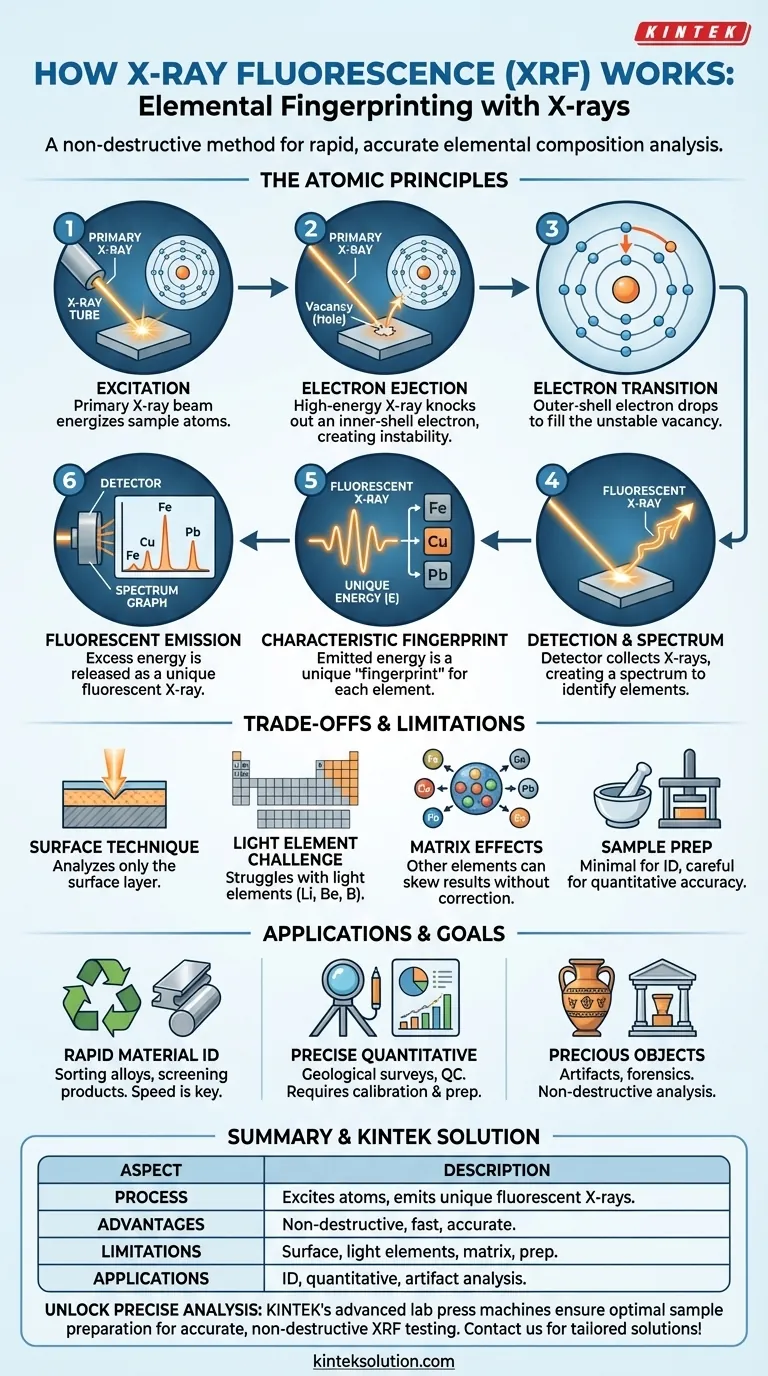
The Atomic Principles of XRF
To understand how XRF works, we must look at the process on an atomic level. The entire technique is based on a predictable, multi-step interaction between X-rays and the electrons orbiting an atom's nucleus.
Step 1: Excitation with a Primary X-ray
The process begins when the instrument, typically using an X-ray tube, directs a high-energy beam of primary X-rays onto the sample material.
Step 2: Ejection of an Inner-Shell Electron
When a primary X-ray strikes an atom in the sample, it can transfer enough energy to knock an electron out of one of its inner orbital shells (most commonly the K or L shell). This ejection leaves a vacancy, or "hole," rendering the atom unstable.
Step 3: The Electronic Transition
An atom cannot remain in this high-energy, unstable state. To regain stability, an electron from a higher-energy outer shell (such as the L or M shell) immediately drops down to fill the vacancy left in the inner shell.
Step 4: Emission of a Fluorescent X-ray
The electron moving from an outer shell to an inner shell has a surplus of energy. This excess energy is released in the form of a secondary X-ray, also known as a fluorescent X-ray.
Step 5: The Characteristic "Fingerprint"
This is the most critical step for analysis. The energy of the emitted fluorescent X-ray is equal to the difference in energy between the outer and inner electron shells. Because the energy levels of these shells are unique to each element, the emitted X-ray has a characteristic energy that acts as a definitive "fingerprint" for that specific element.
Step 6: Detection and Spectrum Analysis
A detector within the XRF instrument collects these emitted fluorescent X-rays. It measures the energy of each X-ray and counts how many are received at each energy level. This data is then plotted on a spectrum, which shows distinct peaks corresponding to the elemental fingerprints of the atoms present in the sample.
Understanding the Trade-offs and Limitations
While powerful, XRF is not without its limitations. Understanding these trade-offs is key to interpreting its results correctly.
It Is Primarily a Surface Technique
The primary X-rays can only penetrate a limited depth into the sample. Therefore, the analysis primarily reflects the composition of the material's surface, which may not be representative of the bulk material if it is not homogeneous.
The "Light Element" Challenge
XRF struggles to detect very light elements (such as lithium, beryllium, and boron). The fluorescent X-rays emitted by these elements have very low energy and are often absorbed by the air or the detector window before they can be measured. While some advanced systems can detect elements as light as carbon, it remains a known challenge.
Matrix Effects
The accuracy of quantitative analysis can be influenced by the "matrix"—all the other elements present in the sample. These other elements can absorb or enhance the fluorescent X-rays from the element of interest, potentially skewing the results if not properly corrected for during calibration.
Minimal vs. Ideal Sample Preparation
One of XRF's greatest advantages is that it requires minimal sample preparation for qualitative identification. However, for the most precise quantitative results, careful preparation (such as grinding a solid into a fine powder and pressing it into a pellet) is often necessary to ensure homogeneity and minimize matrix effects.
How to Apply This to Your Project
Your analytical goal will determine how you leverage XRF technology.
- If your primary focus is rapid material identification: XRF is ideal for its speed and non-destructive nature, providing nearly instant qualitative results for tasks like sorting metal alloys or screening consumer products.
- If your primary focus is precise quantitative analysis: You must use proper calibration standards and may need to perform careful sample preparation to mitigate matrix effects and achieve high-accuracy results for applications like geological surveys or quality control.
- If your primary focus is analyzing precious or unique objects: XRF's non-destructive quality is its greatest strength, allowing you to determine the elemental composition of historical artifacts, artwork, or forensic evidence without causing any damage.
By understanding this process, you can confidently leverage XRF as a powerful tool for unlocking the elemental makeup of your material.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Uses primary X-rays to excite atoms, emitting fluorescent X-rays with unique energies for each element. |
| Steps | 1. Excitation 2. Electron ejection 3. Transition 4. Fluorescent emission 5. Fingerprinting 6. Detection |
| Advantages | Non-destructive, rapid, accurate elemental identification without sample damage. |
| Limitations | Surface analysis, struggles with light elements, matrix effects, requires prep for high accuracy. |
| Applications | Material identification, quantitative analysis, artifact testing in labs and industries. |
Unlock precise elemental analysis for your laboratory with KINTEK's advanced lab press machines! Whether you're preparing samples for XRF with our automatic lab presses, isostatic presses, or heated lab presses, we ensure optimal results for accurate, non-destructive testing. Serving diverse laboratory needs, our equipment enhances efficiency and reliability in material analysis. Contact us today to discuss how KINTEK can support your projects and deliver tailored solutions!
Visual Guide
Related Products
- Laboratory Hydraulic Press 2T Lab Pellet Press for KBR FTIR
- Laboratory Hydraulic Press Lab Pellet Press Button Battery Press
- Automatic Laboratory Hydraulic Press Lab Pellet Press Machine
- Laboratory Hydraulic Split Electric Lab Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Press
People Also Ask
- Why is sample uniformity critical when using a laboratory hydraulic press for humic acid KBr pellets? Achieve FTIR Accuracy
- How is a laboratory hydraulic press used in the FT-IR characterization of copper sulfide nanoparticles?
- What role does a laboratory hydraulic press play in carbonate powder prep? Optimize Your Sample Analysis
- Why use a laboratory hydraulic press with vacuum for KBr pellets? Enhancing Carbonate FTIR Precision
- Why must a laboratory hydraulic press be used for pelletizing samples for FTIR? Achieve Precision in Spectral Data



















